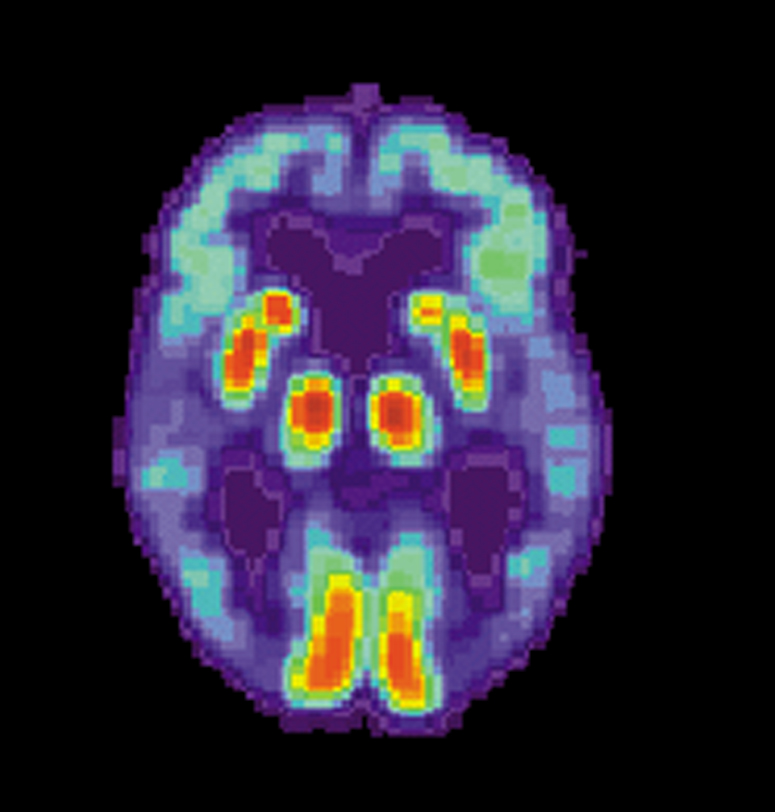
 Washington - A panel of experts consulted by the U.S. agency for the control of food and medicines (FDA) has approved a test that allows for scanning the brain to detect Alzheimer's disease, provided that physicians receive additional training to use it. The procedure involves injecting a blood chemical called Amyvid, manufactured by pharmaceutical giant Eli Lilly, which allows you to highlight, through a scanner, protein deposits that appear to play an important role in disease development.
Washington - A panel of experts consulted by the U.S. agency for the control of food and medicines (FDA) has approved a test that allows for scanning the brain to detect Alzheimer's disease, provided that physicians receive additional training to use it. The procedure involves injecting a blood chemical called Amyvid, manufactured by pharmaceutical giant Eli Lilly, which allows you to highlight, through a scanner, protein deposits that appear to play an important role in disease development.The medical advisory committee voted unanimously on Thursday to recommend the sale of this process on the condition that the laboratory demonstrates that the images from the scanner can be correctly interpreted by physicians trained to do so. The test of Eli Lilly, the first of its kind to be examined by the FDA, is a biomarker called Florbetapit F 18, which is fixed and "light" deposits of amyloid beta-protein so that the scanner can detect them.
The plaques of beta-amyloid appear to be responsible for Alzheimer's because, to accumulate in cortical areas - as seen in autopsies on the brains of people who died from this disease - tend to destroy neurons, which leads to an irreversible degeneration of the brain that affects 26 million people worldwide.
The FDA is not bound to adopt the recommendations of the committee of experts, but usually do it.



No comments:
Post a Comment